Showcase
Manufacture of disposable medical products
A world-renowned manufacturer of medical equipment is focussing its attention on the production of plastic disposable products at one of its locations in Switzerland.
-
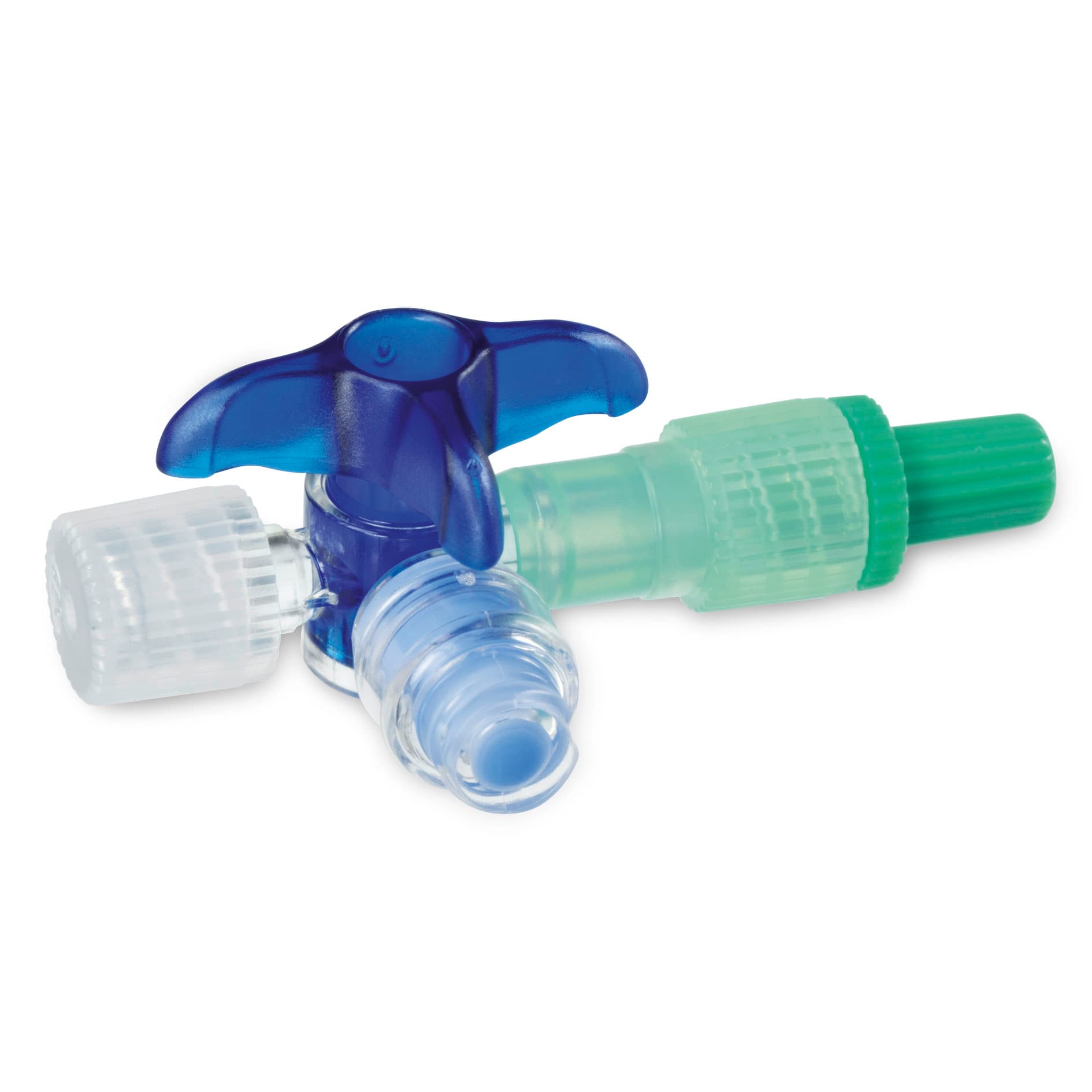
Three-way tap in lipid-resistant plastic used in intravenous therapy (Photo: B. Braun) -

Injection moulding machine for the manufacture of plastic components used in intravenous therapy -

Between one and four temperature control units are installed at each IMM. -

B. Braun’s Escholzmatt production site in Switzerland (Photo: B. Braun)
A world-renowned manufacturer of medical equipment is focussing its attention on the production of plastic disposable products at one of its locations in Switzerland. Manufacturing is taking place continuously in a threeshift operation. In order to ensure consistently high product quality and as little waste as possible, the company is directing particular attention to mould temperature control, a field in which it is cooperating with a Swiss-based manufacturer of temperature control units. The partners’ future plans also include integration of temperature control units into injection moulding machine control.
If a company is so well known in specialist circles that even product designations are actually based on its own name, then we are entitled to assume that we are dealing with a trend setter within the particular segment. B. Braun is Europe’s leading supplier of medical products, product systems and medical services. When it launched the world’s first single-part indwelling venous cannula in 1962, the device was simply referred to as the “Braunüle”. Based in the small town of Melsungen in Hessen, B. Braun’s reputation has now spread far and wide. Today it employs 58,000 staff in 64 countries across all continents. The group has maintained a subsidiary in Switzerland since 1973, and its cooperation with HB-Therm of St. Gallen in the field of temperature control also dates back to this time.
Quality, precision and cleanliness are all top priorities in the manufacture of medical products. The whole production cycle consists of a series of vital quality cogs, all of which need to mesh in order to end up with a perfect medical device. During the entire history of the whole group, which now stretches back for a period of almost 180 years, B. Braun has never lost sight of this vital requirement.
Involvement in Switzerland
Together with many other basic principles, this fundamental premise also underlies the commitment shown by B. Braun to Switzerland as a location. Everything began in Escholzmatt in 1973 at a time when only ten people were employed in the manufacture of disposable plastic medical products. However, development proved to be continuously positive virtually from the outset. Production increased on an ongoing basis, and a facility extension over a footprint of 15,700 square metres was established as early as 1986. The premises in Escholzmatt are still standing today, and in 2005 were extended once more to provide an extra 8,150 square metres of space. The most recent expansion of the manufacturing and administrative site took place in 2016, and its total area now stands at 31,000 square metres. Alongside Escholzmatt, B. Braun’s Swiss-based subsidiary boasts three further locations in Crissier, Lucerne and Sempach. These offer infusion solutions, a central sterilisation plant and disinfectant and hygiene products respectively. In addition, Sempach is home to the company’s Registered Office in Switzerland.
Disposable medical products from Escholzmatt
IIn Escholzmatt, the company focuses on the manufacture of plastic disposable medical products. Its portfolio includes perfusor syringes in various designs, port systems for drip bags and infusion accessories such as three-way taps and valved manifolds. In turn, Escholzmatt supplies the group. This means that most products will find their way back to the company’s central warehouse in Melsungen, Germany. The end consumers are hospitals and clinics all over the world. Because the B. Braun Group manufactures 95 percent of its own products, quality requirements are extremely high. Madeleine Stöckli, CEO of the Swiss-based company B. Braun is also one of the most important aspects within the production process in Escholzmatt itself. We are completely committed to the constant enhancement of all processes.
Measures deployed for this purpose encompass quality certifications, ongoing and systematic process improvement and permanent quality control of all raw materials, production procedures and end products.”
Plastics is a good case in point. The granulate needed as the basis for the whole of the manufacturing chain at Escholzmatt is sourced exclusively from certified suppliers. The final product is thus traceable all the way back to the raw material originally used.
Cooperation with manufacturer of temperature control units
The material processed and produced in Escholzmatt from the very beginning also laid the foundations for cooperation with HB-Therm. Indeed, cooperation arrangements have been in place ever since B. Braun entered the Swiss market.
Injection moulding in Escholzmatt uses Series 4 and Thermo-5 machines from HB-Therm in St. Gallen. This temperature control equipment operates continuously across three shifts to ensure the very highest component quality. More than 250 temperature control units are deployed across the whole of the B. Braun Melsungen Group’s production operations.
Three-way tap with high stress crack resistance
One of the speciality products manufactured in Escholzmatt is Discofix C, a plastic three-way tap component used in intravenous therapy to open or close a connection to more than one infusion medium. The particular property of this part is its high degree of stress crack resistance. This makes it extremely durable against aggressive drugs such as those used in the treatment of cancer. Because of this and other characteristics, Discofix C was awarded an Innovation Recognition Prize by the Central Switzerland Chamber of Commerce in 2001.
In order to ensure output of a consistently high quality, temperature within the mould needs to be precisely managed and maintained. Achieving cost-effective production across a multi-shift system with low levels of manpower and during zero-personnel periods means that rejects must be avoided.
“Because the HB-Therm machines work extremely reliably and deliver unique combined benefits, we deploy them across all our production processes,” states Samuel Bertschi, Head of Innovation. “Monitoring of flow rate as well as main and return line temperatures mean that HB-Therm units are ideally suited to a high-end production facility.
A completely closed cooling circuit is a further advantage. This enables the units to be used in a clean room manufacturing environment directly next to the machine, thus providing even better operational convenience and access. Therefore, these temperature control units allow us to maintain and enhance product quality.”